Introduction: Invasive fungal infections (IFI) are a major threat in patients with hematological malignancies with substantial associated morbidity. Patients who receive CD19 CAR T-cell therapy have multiple immune defects that may predispose them to IFI. However, the role and duration of antifungal prophylaxis in this population is debated.
Methods: This retrospective study at Memorial Sloan Kettering Cancer Center (MSK) describes the characteristics of antifungal prophylaxis and the frequency of IFI after CD19 CAR T-cell therapy in patients with R/R B-cell lymphoma. Two groups of patients were analyzed. Group A (2016-2020): patients primarily received fluconazole, as clinically indicated, regardless of clinical risk profile; Group B (2020-2023) standard guidelines were established in 2020 with antifungal prophylaxis recommended only in high-risk patients with prolonged neutropenia (≥ 3 weeks), those on systemic steroids (≥0.5 mg/kg/day of prednisone equivalent) for ≥ 3 days, or in patients with previous IFI; patients were treated based on a more restrictive mold-active and patient-tailored prophylactic strategy.
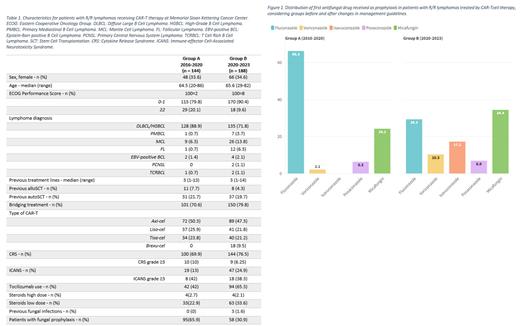
Results: Three hundred thirty-two patients treated with CAR T-cell therapy for R/R lymphoma between 2016 and 2023 were analyzed. Group A was comprised of 144 patients, whereas group B had 188 patients. Patients' characteristics are described in Table 1. Overall antifungal prophylaxis was prescribed in 95 (66.4%) and 58 (30.9%) patients in group A and B, respectively. While fluconazole was most frequently given in both groups, there was 50% less use in group B (Figure 1). Anti-mold azoles were prescribed in 8.4% of group A patients, whereas for group B, it increased to 34.5%. In group A, a switch to another antifungal was observed in 42 (29%) patients, mainly to micafungin because of transaminitis with azoles. In group B, 21 (11.2%) patients switched their first antifungal drug, mainly from fluconazole or micafungin to a mold-active agent following updated guidelines. Median duration of grade IV neutropenia was 9 days (range 1-93) for group A and 12 days (range 1-90) for group B. Grade IV neutropenia was present at the start of prophylaxis in 30 (31.5%) patients in group A and 30 (51.7%) patients in group B. In the subgroup of patients without prophylaxis, only 13% in group A and 4% in group B were neutropenic. Bridging therapy was administered in 70% of patients in group A and 80% in group B. IFI infections after CAR T-cell therapy were rare: one patient had cryptococcal meningoencephalitis in group A (0.7%) and one had invasive aspergillosis in group B (0.5%), both on micafungin prophylaxis, not effective on molds and cryptococcus. These results show better rates for IFI compared to the FDA adverse events reporting system (FAERS) database: which is 3.1% considering the same CAR-T products. All-cause mortality was similar between both groups (A: 29.8% vs B: 35.7%).
Conclusion: This large single-center cohort of R/R lymphoma patients after CAR T-cell therapy offers a valuable snapshot of two different antifungal prophylactic approaches with remarkably low rates of IFI in both strategies, even after the 2020 guideline update with more restrictive antifungal use. Given the risk of toxicity, drug-drug interactions, and concerns for emerging antifungal resistance with azoles, a restrictive prophylactic strategy seems the best choice in this setting.
Disclosures
Melica:Pfizer: Honoraria, Research Funding; Janssen: Honoraria; Gilead: Honoraria, Research Funding. Giralt:Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding; Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees. Palomba:GarudaTherapeutics: Honoraria; Smart Immune: Honoraria; Thymofox: Honoraria; Novartis: Honoraria; Ceramedix: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Rheos: Honoraria; Juno: Honoraria, Patents & Royalties; Cellectar: Honoraria; BMS: Honoraria; MustangBio: Honoraria; Kite: Honoraria; Pluto Immunotherapeutics: Honoraria; Synthekine: Honoraria. Park:Intella: Consultancy; Servier: Consultancy, Research Funding; Allogene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Minerva Bio: Consultancy; BeiGene: Consultancy; Incyte: Research Funding; Bright Pharmacetuicals: Consultancy; Pfizer: Consultancy; Be Biopharma: Consultancy; Sobi: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Amgen: Consultancy; Fate Therapeutics: Research Funding; Autolus Therapeutics: Research Funding; Genentech, Inc.: Research Funding; Kite: Consultancy; Curocell: Consultancy; Artiva Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Affyimmune: Consultancy; GC Cell: Membership on an entity's Board of Directors or advisory committees. Salles:Ipsen: Consultancy, Research Funding; Loxo/Lilly: Consultancy; Genmab: Consultancy; Merck: Consultancy, Honoraria; Nordic Nanovector: Consultancy; Kite/Gilead: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Orna: Consultancy; AbbVie: Consultancy, Honoraria; ATB Therapeutics: Consultancy; Molecular Partners: Consultancy; Incyte: Consultancy; Janssen: Consultancy, Research Funding; EPIZYME: Consultancy; Owkin: Current holder of stock options in a privately-held company; Debiopharm: Consultancy; BMS/Celgene: Consultancy; BeiGene: Consultancy; Novartis: Consultancy; Nurix: Consultancy. Scordo:Medscape, LLC: Honoraria; CancertNetwork (Intellisphere LLC): Honoraria; Omeros Corporation: Consultancy, Research Funding; Amgen, Inc.: Research Funding; Angiocrine Bioscience, Inc.: Research Funding. Shah:ArcellX: Other: DSMB; Janssen: Research Funding; Beyond Spring: Research Funding; BMS: Research Funding; Amgen: Research Funding. Perales:Allogene: Research Funding; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; MorphoSys: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; AbbVie: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; Syncopation: Honoraria; Cidara Therapeutics: Consultancy, Other; NexImmune: Consultancy, Current equity holder in publicly-traded company; Medigene: Consultancy, Other; Equillium: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Vor Biopharma: Consultancy, Honoraria; Allovir: Consultancy; Kite: Consultancy, Honoraria, Research Funding; Servier: Other; Miltenyi Biotec: Honoraria; DSMB: Other; Incyte: Consultancy, Honoraria, Research Funding; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; Adicet: Honoraria; Celgene: Honoraria; Karyopharm: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Sellas Life Sciences: Consultancy; VectivBio AG: Consultancy, Honoraria; Takeda: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal